Drug R&D productivity declines
The introduction of a new drug to the market is long and costly, taking 10-15 years and requiring $1 to $3 billion. Not only that, but throughout the drug development journey, a therapeutic agent faces a significant 90% probability of failure due to issues related to pharmacokinetics, efficacy, or off-target-associated toxicity [1].
In response to this challenge, the pharmaceutical industry continually refines each phase of the drug development process, driving remarkable advancements in both computational and biological sciences. This progress has led to the identification of new druggable targets, in particular for RNA, whose role in cellular functions and gene expression has presented a promising avenue to treat diseases such as cancer and neurodegenerative disorders. Within this evolving landscape, our SYNSIGHT talent team is at the forefront, taking advantage of both an innovative biological approach and state-of-the-art artificial intelligence solutions, with a focus on the emerging field of RNA targeting. Our strategy promises the discovery of innovative, next-generation drugs in a fast and cost-efficient fashion.
AN INNOVATIVE CELL BASED ASSAY FOR NEW SOLUTIONS
SYNSIGHT develops an exclusive, patented technology to detect in cellulo interactions: the Microtubule Bench assay: MTbench®, originated from INSERM (French national institute of health) [2],[3].
The MTbench® assay uses the microtubule network as an intracellular bench to detect protein interactions or RNA-protein interactions in living cells, and use cellular machinery to deploy the assay setup. The MTbench® technology therefore enables the direct description of RNA-protein or protein-protein interactions in a live cellular context. Moreover, inhibition/promotion of the interaction by pharmaceutical agents is measurable, enabling high-throughput screening of these interaction modulators. In the last few years, we developed, optimized, and standardized the MTbench® technology using lab automation and robotics, and enlarged the spectrum of pharmaceutical applications. MTbench® is easy to set up and allows for direct visualization within single cells. Its automated and robotized system can screen thousands of compounds, while providing high sensitivity and specifity through the analysis of thousands of cells, detecting activity across nanomolar to micromolar concentrations. The MTbench® screening assay was also coupled to a series of complementary assays, finely characterizing the mode of action of active compounds and excluding undesired activities.
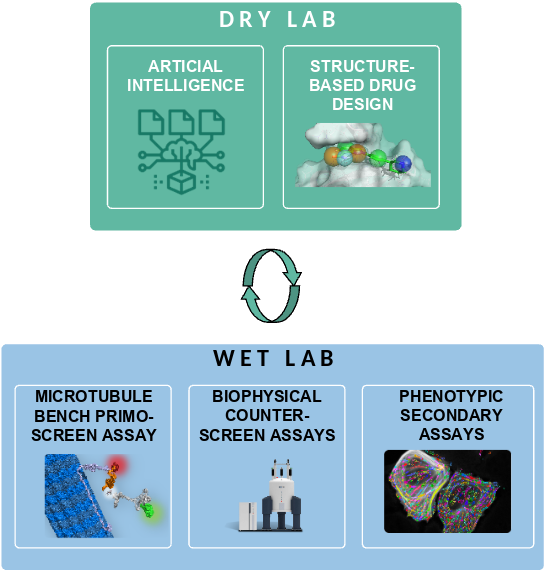
OPTIMIZED ITERATIVE CYCLE
At SYNSIGHT, we design small molecules by tackling machine learning, chemoinformatics and data science to develop and implement intelligent algorithms. Our algorithms serve the purpose of swiftly exploring chemical landscapes and, with growing efficiency, identifying bioactive agents suitable for inclusion in RNA-targeting screens. Current target- and ligand-based in silico techniques greatly increase the likelihood of finding a promising compound within a limited set of tested agents during in cellulo screening [4].
But combining the mentioned techniques with artificial intelligence offers us a great advantage: ongoing enhancement of our chemoinformatic platform, continually fed with an increasing volume of robust and rich experimental results from the bench [5]. Hence, our vision is to steadily elevate screening success rates, outperforming conventional drug discovery strategies. This achievement, applied to our internal initiatives, collaborations, or service projects, founds our strategy for accelerated and more reliable RNA targeting drug discovery.
The resulting screening cascade produces key activity data, from the atomic up to the phenotypic scale. These data are then treated by our computational chemists, who produce an upgraded selection of new compounds injected into our iterative selection workflow.
Conclusion
Our cutting-edge approach combines lab automation, innovative cell-based screening technologies, in silico techniques, and AI-driven insights to improve and accelerate the selection of RNA-targeting drug-like compounds. We use generative models to design new molecules and virtually screen them before they get tested at the bench in a combination of cell-based and biophysics assays. A key aspect of this approach is we can target a specific interaction of a specific structural pocket present in any protein of interest, allowing us to finely tune key interactions filling stringent druggability criteria. The synergy between our highly skilled scientists from biology, chemistry and computer science will unlock higher screening success rates, outperforming conventional drug discovery strategies, and thereby reducing the rate of drug development failures. Our mission is to bridge the gap between innovative in silico drug design and robust in cellulo screening, ultimately advancing the field of RNA-targeting drug discovery.
By combining cutting-edge technologies and expertise, we strive to make a significant impact on preclinical cost-efficiency and predictive pharmaceutical investigations, leading to the development of more effective and safe therapeutic agents for various diseases.
[1] Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of Clinical Drug Development Fails and How to Improve It? Acta Pharm Sin B 2022, 12 (7), 3049–3062. https://doi.org/10.1016/j.apsb.2022.02.002.
[2] Boca, M.; Kretov, D. A.; Desforges, B.; Mephon-Gaspard, A.; Curmi, P. A.; Pastré, D. Probing Protein Interactions in Living Mammalian Cells on a Microtubule Bench. Sci Rep 2015, 5, 17304. https://doi.org/10.1038/srep17304.
[3] Maucuer, A.; Desforges, B.; Joshi, V.; Boca, M.; Kretov, D. A.; Hamon, L.; Bouhss, A.; Curmi, P. A.; Pastré, D. Microtubules as Platforms for Probing Liquid–Liquid Phase Separation in Cells – Application to RNA-Binding Proteins. J Cell Sci 2018, 131 (11). https://doi.org/10.1242/jcs.214692.
[4] Chang, Y.; Hawkins, B. A.; Du, J. J.; Groundwater, P. W.; Hibbs, D. E.; Lai, F. A Guide to In Silico Drug Design. Pharmaceutics 2023, 15 (1), 49. https://doi.org/10.3390/pharmaceutics15010049.
[5] Han, R.; Yoon, H.; Kim, G.; Lee, H.; Lee, Y. Revolutionizing Medicinal Chemistry: The Application of Artificial Intelligence (AI) in Early Drug Discovery. Pharmaceuticals 2023, 16 (9), 1259. https://doi.org/10.3390/ph16091259.